Nh3-nh3 because they are both polar molecules. Experts are tested by Chegg as specialists in their subject area.
Which pair of molecules has the strongest dipole-dipole interactions.
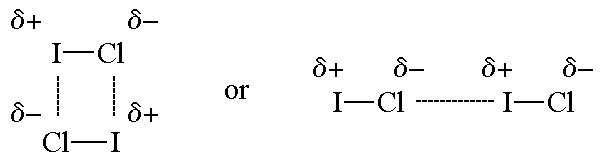
. The covalent bond that holds atoms together within a molecule or the electrical attraction between two neighboring. A Dipole-dipole attraction is a weaker force than covalent bonds being about 50 as strong. D CH4 and CH4.
Among the given pairs of molecules NH3 AND NH3. NH3 and CHA D. Dimethyl ether 248 K e.
The closer ion and polar molecule are the stronger the intermolecular force is between polar molecule and ion. An ion with higher charge. NH_ 3 molecule causing strong dipole-dipole interactions.
CO2 and CO2 B. Nitrogen is third most electronegative and hydrogen is highly electropositive and thus strong dipoles develop in. 119 A CH 4 and CH 4 B CO 2 and CO 2 C NH 3 and NH 3 D CO 2 and CH 4 E NH 3 and CH 4.
Which pair of molecules has the strongest dipole-dipole interactions. 119 Which pair of molecules has the strongest dipole - dipole interactions. What pair of molecules has the strongest dipole - dipole interactions co2-co2 or co2-ch4 or nh3-nh3 or nh3-ch4 or ch4-ch4.
A NH3 and CH4. Put in order from strongest to weakest. Which has the strongest dipole.
Sodium chloride NaCl Which is stronger. B Dipole-dipole attractions increase as the. C2H6O is the empirical formula for both ethyl alcohol CH3CH2OH.
Which pair of molecules has the strongest dipole-dipole interactions. Pair has two highly polar molecules that are even able to form hydrogen bonds with each other which is an extreme. Propane 231 K d.
B NH3 and NH3 C CO2 and CO2. CO and CHA E. How can you tell which dipole-dipole force is stronger.
Based on their boiling points which of the following compounds has the largest dipole-dipole interaction. Order the intermolecular forces dipole-dipole London dispersion ionic and hydrogen-bonding from weakest to strongest. We review their content and use your feedback to keep the quality high.
D NH 3 and NH 3. Generallyi Cis - isomer has more dipole moment than trans - isomerii Cis is less stable than transiii The boiling points of cis isomers are more than those of trans isomers remember. London Dispersion Forces Hydrogen Bonds Dipole-dipole interactions ionic.
A NH and CH B NH and NH C CO and Co D CH and CH fram fosfase n Solve Study Textbooks Guides. Methyl chloride 249 K b. CHA and CH C.
100 6 ratings Transcribed image.
Out Of Bcl3 And So2 Which Compound Exhibits Dipole Dipole As Its Strongest Force Why Quora

The Gases Used In The Spark Discharged Apparatus Were

Solved Which Pair Of Molecules Has The Strongest Chegg Com

Which Pair Of Molecules Has The Strongest Dipole Dipole Interactions
0 Comments